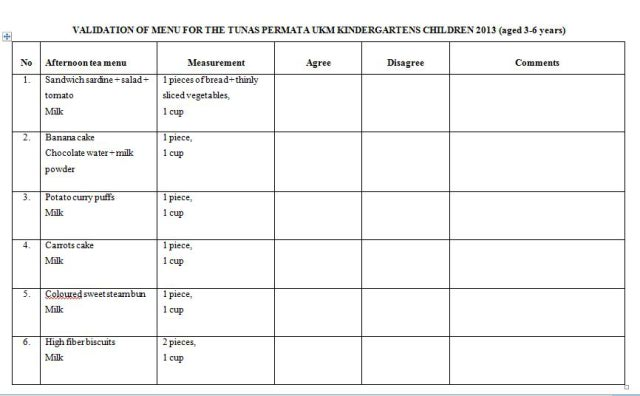
Should we be worried about our pH control?
Question
I am interested in the control of body Ph.
I have seen a lot of information about it online -
mostly selling liquids or pills.
Please give me your thoughts on this.
I read this: (Link to page included)
-----------------------------------
http://www.byebyecarbs.com/acid-base-dietary-balance-is-a-scam/
The pH in Body Fluids is 7.4, Plus or Minus 0.02
It抯 regulated at that level, but the primary site of pH control is in the cells and pH dictates enzyme activity. Here the pH is held constant at 7.0 and any disruption is rapidly counteracted so the pH remains constant.
The addition of either acids or alkaline substances to the body is immediately adjusted so that pH is strongly defended. The idea that you can change your pH is absurd ?you can抰.
The silliness continues when the zealots argue the an acid pH is conducive to getting cancer and other diseases. One should therefore consume an alkaline diet and this suggests that alkaline foods will help maintain a pH in the range of 7.4. Well, that抯 what the body will do or it will die. The failure to maintain tight control over pH will lead to disease.
But the body is programmed to maintain this control and when the body can longer do this, it is because of a disease process. You cannot control in-born body functions, this is called homeostasis. People will next argue that one can control his temperature level. Some processes are under very tight control and have very narrow ranges.
Others, like bodyweight can range widely but still be compatible with life.
Forget the acid-alkaline nonsense
-----------------------------------
Thanks,
Jim Furr ><>
Dear Jim,
The quotation does make perfect sense. Body's pH indeed is tightly controlled. If pH the blood decreases too much causing what is called acidemia, the body counteracts by increasing breathing to remove CO2, so that less H ions became free; thus the pH will rise back to normal. For alkalemia, when pH goes too high, the opposite process also results in setting pH back to normal. There are pathological states when the body fails to compensate, this happens, for example, during diabetic ketoacidose, when excess ketone bodies (normally a good energy source for the body and the brain) cannot be utilized and accumulate in the blood.
Tanya Zilberter
- Prev:Making exercise fun
- Next:Working Out
Related Articles
-
Absorption of heme iron versus non-heme iron
QuestionThanks for you earlier response Tanya. Can you help me u
-
Low fat, low calorie dieting: is there a success?
Questionhello, i am 26 year old married woman. my weight is 130L
-
coconut
QuestionHi I come from a part of India where coconut and coconut
-
New Way of Eating
QuestionGood afternoon, I am 15 years old, 5 feet a
-
Increase triglyceride levels
QuestionDear Todd, Im 15 and an elite basketball player(64, 160 p
-
Gradually Decreasing Weight
Question A few months ago, I went off to university, and have fou