Nervous About Diet Pills? You Should Be!
Prescription Drugs:
Fen-Phen was removed from the market by the FDA in 1997 because it may have been causing pulmonary hypertension and heart valve problems. Fenfluramine the Fen part of Fen-Phen and its stereoisomer dexfenfluramine were introduced as single drugs but they too were retired from the market by the FDA in 1997. The related drug benfluorex was removed from the market in France in November 2010 due to concerns over thickening of heart valves.
The antidepressant, bupropion, has not been permitted by the FDA for weight loss. The combination of bupropion and naltrexone, an opioid blocker, developed under the brand name Contrave is pending final decision by the FDA. The FDA advisory committee has raised concerns over possible adverse effects on blood pressure in some individuals. Clinical trials demonstrate that half of the individuals in the trials lost an additional 5% of their body weight in year-long studies.
Phentermine, related to amphetamines, is referred to as an anorectic and has been approved for weight loss by the FDA for no more that 3 consecutive months. Side effects include hypertension and irregular heartbeats. The combination of phentermine and topiramate under the brand name Qnexa was rejected by FDA on Oct. 29, 2010. Side effects of the combination drug include psychiatric and cognitive impairment.
Similarly Qnexa and Lorcaserin were also rejected by FDA on Oct. 23, 2010. Side effects include psychiatric and cognitive impairment as well as cardiovascular events.
Sibutramine, an appetite suppressant, was approved by the FDA for use in weight loss for up to 2 years but was recently pulled from the market by its manufacturer pending a review of current data on side effects. Sibutramine has been implicated in an increased risk of hypertension, irregular heartbeats, non-fatal heart attacks and stroke.
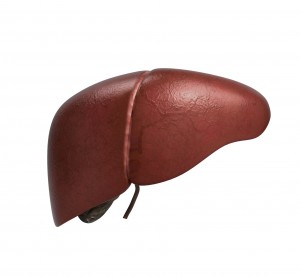
The digestive enzyme inhibitor, Orlistat sold in prescription strength as Xenical and over the counter as Alli, was approved by the FDA for use in weight loss over a decade ago. Until recently the side effects were limited to the malabsorption-like symptoms of oily discharge, bloating, flatulence and a particular form of diarrhea termed steatorrhea. However, the FDA recently issued an order that these products carry a warning label because of an increased risk of liver disease and possibly colon and breast cancer.
Natural Supplements:
Although implicated in some deaths and once banned by the FDA the amphetamine-like substance ephedra or ma wong is still widely available on the internet. Side effects include hypertension and heart attack.
Meta-analysis of the “fat-blocker” chitosan has shown it to have no effect on weight loss in humans.
Similarly, there is no clinical evidence to support the claim that hoodia is an effective appetite suppressant that promotes weight loss.
There are a wide range of diuretics and “cleanses” that cause short term weight reduction due to water loss but these effects are transient. As are the effects of the once popular body wraps.
The naturally occurring, soluble, dietary fiber in Mirafit fbcx has been demonstrated to be effective for both weight loss and weight maintenance in published clinical trials. Taken with fat-containing meals, as directed, six tablets per day will reduce fat absorption by about 50-60% which accounts for about 500 calories/day or 1-1 ? pounds per week. The side “benefits” include a decrease in triglyceride and both total and LDL (bad) cholesterol levels in those individuals who have a blood fat problem. Mirafit fbcx also increases both insulin and leptin sensitivities so that glucose control and satiety are both improved. Studies conducted at the University of Minnesota and the National Institutes of Health indicate that Mirafit fbcx preferentially binds and removes the unwanted saturated and trans fats.
Confusion Around Clinical Trials:
Typically the clinical trials that are designed to study weight loss involve putting all of the subjects in the study on a calorie restricted diet and then comparing the differences between the active and placebo groups. Consequently the results are usually stated in terms like, “.at the end of the one-year study 50% more of the active group lost X% more weight than did the placebo group.”. If you find such language complicated to interpret you are not alone. Without having the study results in front of you and without a whole lot of experience in interpreting data it is very difficult for anyone to understand such statements. The Mirafit fbcx studies are different; we took the position that if you have the will power to go onto a calorie restricted diet for a year then you really do not need to rely on a pill to help you. Consequently, our study subjects were asked not to change their diet or exercise regime during the investigation so that weight loss can easily be interpreted as being the result of the product.
This article comes to you from the creators of Mirafit fbcx , the naturally occurring soluble dietary fiber that binds and eliminates 500 calories per day.
-
Easy To Find Weight Loss Supplements That Work
It seems that all around us, people are trying to get in shape, slim
-
Top Tips For Losing Weight With The Slimweight Patch
Whether or not you抮e like such a lot of
-
Dealing With The Results Of Weight Loss
It may seem to be a strange contradiction but there are some people wh
-
5Step Weight Loss Program For Permanent Weight Loss
Every Journey Worth Taking Begins with a Single StepHere are five easy
-
Drinking Yerba Mate for Fat Burning
Yerba mate has shown up in a lot of different supplements lately. If y
-
Diet Plans: Sensible and Simple Choices for Eating
When it comes to consuming a healthy diet, most men and women are c
- DON'T MISS
- Overweight Health Risks
- To Buy HCG Drops Or HCG Injections?
- 30 Minutes To Your Most Amazing Body Yet
- What Is Dynamic IT?
- Enhance Your Exercise Routine With A Ball
- Weight Loss Or Fat Loss Important Differences To Lose Weight And Fat Healthily
- The Cookie Diet for Weight Loss?
- How to Set Sensible Weight Loss Goals
- How to Get Up in the Morning to Exercise
- In postmenopausal women, even light physical activities are effective against weight gain